FDA Medication Guide: What You Need to Know About Safe Drug Use
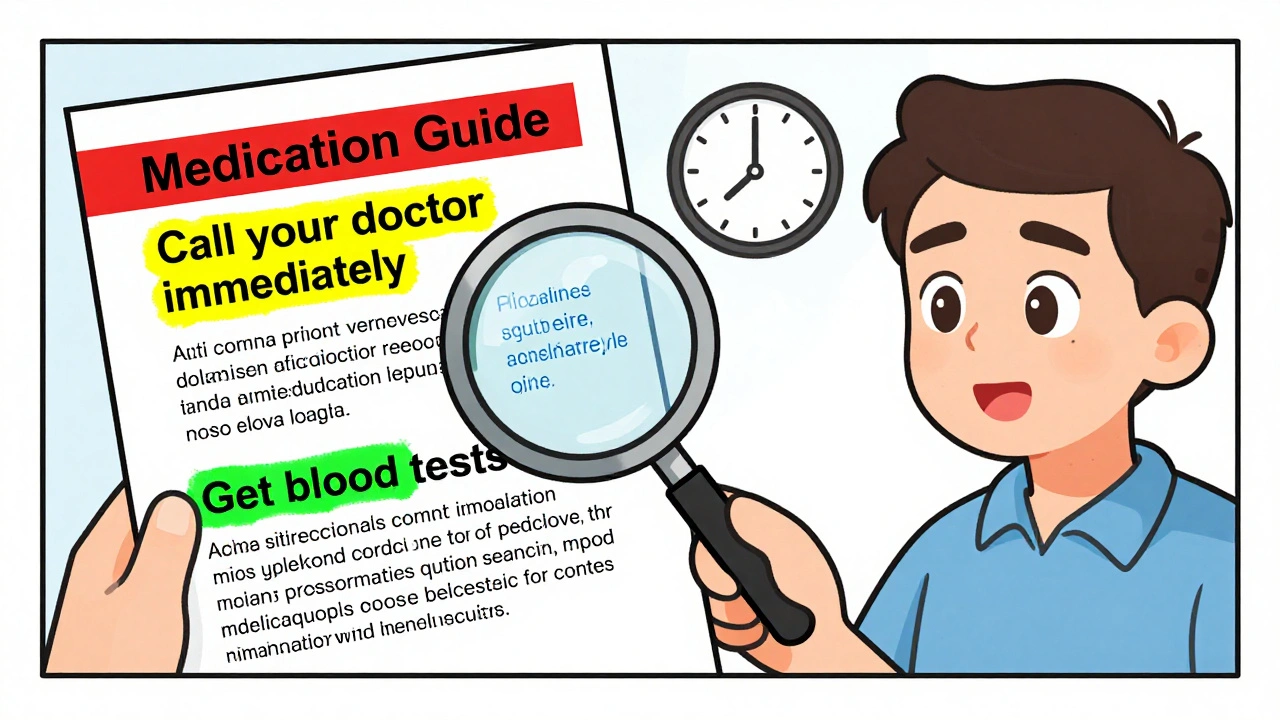
When you pick up a prescription, the FDA Medication Guide, a printed handout required by the U.S. Food and Drug Administration for certain high-risk medications. Also known as a Patient Package Insert, it's meant to give you clear, plain-language facts about serious risks, how to use the drug safely, and what to watch for. But here’s the catch: most people never read it. And that’s where things go wrong. These guides aren’t marketing brochures—they’re legal documents designed to warn you about life-threatening side effects, dangerous interactions, and hidden dangers that even your doctor might not have time to explain.
The FDA Medication Guide, a legally mandated patient safety tool for drugs with serious risks. Also known as Patient Package Insert, it's meant to give you clear, plain-language facts about serious risks, how to use the drug safely, and what to watch for. The real value isn’t in the big warnings like "black box" alerts—it’s in the small print. For example, a guide for a blood thinner might tell you that idarucizumab is the only antidote that works fast enough in an emergency. Or that a generic version of your drug might have different inactive ingredients that cause unexpected reactions. These aren’t hypotheticals. They’re real events tracked by the FDA after drugs hit the market. Post-market studies show that even approved generics can cause problems when batch variability shifts how the drug behaves in your body.
What makes the FDA Medication Guide, a legally mandated patient safety tool for drugs with serious risks. Also known as Patient Package Insert, it's meant to give you clear, plain-language facts about serious risks, how to use the drug safely, and what to watch for. so useful is how it connects to other critical safety layers. It’s not just about the active ingredient—it’s about how your liver processes it, what other meds you’re taking, and even when you take it. A bedtime dose of blood pressure medicine might stop your daytime dizziness. A high-protein meal can block Parkinson’s medication from working. And if you’re fasting for Ramadan, some pills can’t be taken at all without risking harm. These aren’t side notes—they’re part of the FDA’s safety framework, and they’re all buried in those guides.
What you won’t find in the guide? Answers to how much it costs, whether your insurance covers it, or if there’s a cheaper generic. But you will find what happens if you mix it with alcohol, antidepressants, or even certain herbal supplements. That’s why cinnarizine warnings mention sedatives. Why naloxone is listed as a must-have antidote for opioid overdoses. Why the FDA tracks drug shortages and fentanyl contamination after the pandemic broke supply chains. These guides are your first line of defense—not your pharmacist, not your doctor, not Google. It’s the paper they hand you with the pill bottle.
Below, you’ll find real stories from people who ignored these guides—and those who saved themselves by reading them. From reversal agents for blood thinners to how biosimilars compare to originals, from pill splitting tricks to why generic drug trust is still broken. These aren’t theoretical debates. They’re lived experiences. And every one of them ties back to one thing: understanding what the FDA Medication Guide is really trying to tell you.