When you pick up a generic pill at the pharmacy, you’re likely saving money-sometimes half or more compared to the brand name. But have you ever wondered what happens after that drug hits the market? Unlike brand-name drugs, which go through years of clinical trials involving thousands of patients, generic drugs get approved based on one key fact: they’re bioequivalent to the original. That means they deliver the same active ingredient at the same rate and amount. But bioequivalence doesn’t guarantee identical safety in the real world.
Why Generic Drugs Need Extra Monitoring
The FDA approves over 10,000 generic drugs every year. These make up 90% of all prescriptions filled in the U.S. But here’s the catch: most generic drugs are never tested in large, diverse populations before they’re sold. Clinical trials for generics usually involve fewer than 5,000 people-mostly healthy adults, not elderly patients, children, or pregnant women. That’s a problem because side effects can hide until a drug is used by millions with different health conditions, diets, and other medications. For example, a generic version of a blood thinner might work perfectly in a controlled trial. But when thousands of older patients with kidney issues start taking it, unexpected bleeding events might pop up. Or a generic thyroid medication might dissolve slightly slower in some people’s stomachs, leading to inconsistent hormone levels. These aren’t theoretical risks-they’ve caused real harm.How the FDA Tracks Problems After Approval
The FDA doesn’t just approve generics and walk away. They run one of the most advanced drug safety systems in the world: the Sentinel Initiative. Launched in 2008 and fully active by 2016, Sentinel scans health records from over 300 million Americans-hospitals, insurers, clinics-all in near real time. It looks for patterns: spikes in hospital visits, sudden increases in liver enzyme reports, or clusters of allergic reactions tied to a specific generic drug. But Sentinel isn’t the only tool. The FDA also relies on MedWatch, a voluntary reporting system where doctors, pharmacists, and even patients can report side effects. In 2022, over 1.3 million adverse event reports came in through MedWatch. About 1 in 5 involved generic drugs. The problem? Only 35% of those reports named the actual manufacturer. That makes it hard to tell if the issue is with one company’s version-or if it’s a problem shared by all generics of that drug.What Goes Wrong with Generics?
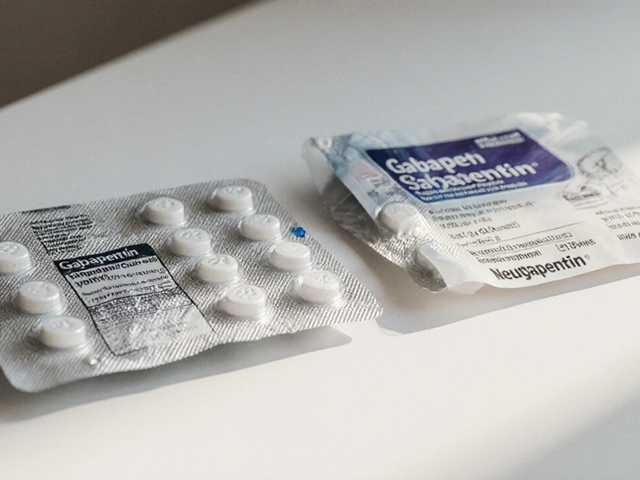
It’s not always about the active ingredient. Sometimes, it’s the fillers, the coating, or how the tablet breaks down in your body. A 2022 FDA analysis found that 78% of all drug recalls that year were for generic medications. Why? Here are the top issues:- Tablet dissolution problems: The pill doesn’t break down fast enough-or too fast-leading to under- or overdosing. This was a major issue with generic levothyroxine, where patients switching between manufacturers reported palpitations, weight changes, and fatigue.
- Transdermal patch failures: Patches that don’t stick properly can deliver inconsistent doses. In 2022, 27% of patch-related safety reports cited poor adhesion.
- Precipitates in liquids: Some generic injectables or oral suspensions form clumps or particles that shouldn’t be there, risking blockages or allergic reactions.
- Manufacturing inconsistencies: A generic made in India might use a different binder than one made in the U.S., even if both meet FDA specs. That small difference can matter for sensitive patients.
Real Stories Behind the Data
A 2021 study in JAMA Internal Medicine found that 68% of serious adverse events linked to cardiovascular generics weren’t listed on the drug label at the time of approval. That means doctors had no warning. One pharmacist in Ohio told of three patients who developed irregular heartbeats after switching from one generic levothyroxine brand to another. All three had stable thyroid levels for years-until the switch. Dose adjustments were needed. No one knew why-until they traced it to a different filler in the new version. On Reddit, a thread from June 2023 with over 140 comments featured pharmacists sharing similar cases. One wrote: “I’ve had four patients in six months report nausea and dizziness after switching to a new generic of metformin. All symptoms stopped when we switched back.” But it’s not all bad. A 2023 Kaiser Family Foundation survey found that 89% of patients on generics for high blood pressure or diabetes reported no issues at all. The cost savings allow people to stick with their meds-which is a huge win.Who’s Responsible for Monitoring?
Generic manufacturers are legally required to report any adverse events to the FDA. Serious ones-like hospitalizations or deaths-must be reported within 15 days. Others go in quarterly. But here’s the challenge: many small generic companies don’t have the staff or tech to do this well. A 2022 Tufts study found the average annual cost to run a full pharmacovigilance system is $1.2 million. That’s a lot for a company selling only a few low-margin drugs. Large manufacturers like Teva, Mylan, and Sandoz use AI tools to scan reports and spot signals early. But smaller ones still rely on manual checks. That’s why the FDA stepped in. In 2021, they issued a warning letter to Teva for failing to report hundreds of adverse events on time. The result? A six-month delay in approving new generic products.
The Gap Between Regulations and Reality
The FDA says all generics must follow the same safety rules as brand-name drugs. And technically, they do. But the system has blind spots. One major issue: the same generic drug can be made by five different companies. If a patient has a bad reaction, they rarely know which version they took. Pharmacists don’t always know either-unless they check the label closely. The Government Accountability Office (GAO) reported in May 2023 that inconsistent manufacturer reporting is the biggest barrier to catching safety problems. Without knowing which company’s product caused the issue, the FDA can’t target inspections or recalls effectively. That’s why new tools are coming. The FDA’s 2024-2026 plan includes developing “product-specific surveillance plans” for high-risk generics-like inhalers, injectables, and patches. They’re also testing blockchain systems to track each batch from factory to pharmacy. Five major generic makers are already running pilot programs.What You Can Do
If you’re on a generic drug, especially one with a narrow therapeutic index (like warfarin, levothyroxine, or seizure meds), here’s what matters:- Check the label. Note the manufacturer name. If you refill and the pill looks different, ask your pharmacist if it’s the same maker.
- Track symptoms. If you feel worse after switching generics-fatigue, dizziness, rash, irregular heartbeat-don’t brush it off. Tell your doctor and ask if it could be the medication.
- Report it. You can file an adverse event report with the FDA through MedWatch. Even if you’re not sure, your report could help spot a pattern.
- Stick with one brand. If a specific generic works for you, ask your doctor to write “dispense as written” on the prescription. That stops the pharmacy from switching without your knowledge.
The Bottom Line
Generic drugs are safe for most people-and they save billions in healthcare costs every year. But safety doesn’t end at approval. The system that monitors them is complex, under-resourced, and still catching up to the scale of the market. The FDA is improving, using data and tech to find problems faster. But the real power lies with patients and providers who notice the subtle changes and speak up. Post-market surveillance isn’t flashy. It doesn’t make headlines. But it’s the quiet guardrail keeping millions safe. And it only works if we pay attention.Are generic drugs less safe than brand-name drugs?
Generic drugs are not inherently less safe. They must meet the same FDA standards for quality, strength, and purity as brand-name drugs. But because they’re not tested in large, diverse populations before approval, some safety issues only appear after millions of people start using them. That’s why post-market surveillance is critical-it catches problems that pre-approval trials miss.
Can switching between generic manufacturers cause side effects?
Yes, especially for drugs with a narrow therapeutic index, like levothyroxine, warfarin, or seizure medications. Even small differences in inactive ingredients or how the drug dissolves can affect absorption. Many patients report changes in symptoms after switching manufacturers-even when the generic is labeled the same. If you notice new side effects after a switch, talk to your doctor and pharmacist.
How does the FDA know if a generic drug is unsafe?
The FDA uses multiple tools: the Sentinel Initiative scans electronic health records for safety signals, MedWatch collects reports from doctors and patients, and drug manufacturers are required to report adverse events. AI tools help detect patterns, like a sudden spike in liver damage linked to one specific generic. If a trend emerges, the FDA can issue warnings, request label changes, or order recalls.
Why are so many generic drugs being recalled?
In 2022, 78% of all drug recalls were for generics. Most weren’t due to dangerous ingredients, but to manufacturing issues: tablets that don’t dissolve properly, patches that fall off, or liquids that form clumps. These problems can lead to underdosing or overdosing. The FDA is increasing inspections and pushing manufacturers to improve quality control-especially for complex products like inhalers and injectables.
What’s being done to improve generic drug safety monitoring?
The FDA’s 2024-2026 plan includes developing individual safety plans for high-risk generics, expanding the use of real-world data through the Sentinel system, and testing blockchain to track each batch of generic drugs from factory to pharmacy. They’ve also allocated $15 million under GDUFA III to boost surveillance efforts. The goal is to move from reactive reporting to proactive detection.












9 Comments
Generic drugs are a lifeline for people in developing countries. I’ve seen patients in rural India choose generics because it’s the only way they can afford treatment. The system isn’t perfect, but it’s saving lives every day. We need better tracking, sure-but don’t throw the baby out with the bathwater.
As a pharmacist in Ohio, I’ve seen firsthand how switching generic brands can mess with patients on levothyroxine. One guy went from feeling fine to jittery and sweating-turned out the new version had a different filler. He didn’t even know the pill looked different. Docs need to talk about this more.
OMG I JUST HAD THIS HAPPEN TO ME!! 😱 I switched generics for my blood pressure med and started getting dizzy like crazy. Thought I was going to pass out. My pharmacist said ‘it’s the same thing’-BUT IT WASN’T. I went back to the old one and BOOM, normal again. Why is this even allowed??
generic drugs r the best thing ever for people who cant afford brand names. i got my diabetes meds for $4 a month instead of $400. yeah sometimes the pill looks diff but i dont care as long as it works. if u got issues talk to ur doc not the internet lol
Let’s be real-this whole ‘bioequivalence’ thing is a scam. The FDA is in bed with Big Pharma and generic manufacturers. Why do you think 78% of recalls are generics? Because they’re made in shady factories with dust-covered equipment. They don’t test for real-world use because they don’t WANT to. It’s all about profit, not safety.
Ugh. I knew it. Big Pharma is letting cheap junk get approved so they can keep charging $1000 for the brand name. And now they’re using blockchain? LOL. That’s just to make it look like they’re doing something. They don’t care if you die as long as the stock price goes up. 💀
You’re all missing the point. The FDA’s entire system is broken. They approve generics based on *bioequivalence*-which is a statistical mirage. Real-world pharmacokinetics vary wildly based on gut pH, liver enzymes, even time of day. And you think a tablet dissolving 0.5 seconds slower won’t kill someone on warfarin? Wake up. This isn’t a ‘minor issue’-it’s systemic negligence. And no, ‘reporting side effects’ isn’t a fix. It’s a band-aid on a severed artery.
Oh look, another article about generics that blames the manufacturers while ignoring that 90% of prescriptions are generic because patients *choose* them. The real problem? Doctors who don’t check the manufacturer and pharmacists who switch without telling anyone. You want safety? Ask for the brand. Or better yet-don’t let your pharmacy switch without consent. Simple. No blockchain needed.
Guys, I get it. Switching generics sucks. I had a cousin on levothyroxine who went from 0 to 100 in anxiety after a switch. But here’s the thing-we can’t go back to paying $300/month for every med. The answer isn’t to scrap generics. It’s to demand better tracking. Tag every pill with a batch code. Force manufacturers to report ALL side effects. And yes, if you’re on a critical med, stick with one brand. No shame in that. 💪💊