Cardiovascular Generics: What Safety Studies and Real-World Data Really Show
Cardiovascular generics save billions, but safety data shows mixed outcomes. Learn what studies really say about effectiveness, risks, and when to stick with brand names.
When you pick up a generic drug, a lower-cost version of a brand-name medication that contains the same active ingredient and meets the same FDA standards for safety and effectiveness. Also known as generic medication, it is the backbone of affordable healthcare for millions. But safety isn’t just about whether the pill has the right chemical—it’s about whether you can trust it to work the same way every time, without hidden risks.
Many people worry that generics are cheaper because they’re less effective. That’s not true. Decades of real-world data, including studies from the FDA and European Medicines Agency, show that bioequivalence, the scientific standard proving a generic drug behaves in the body just like the brand-name version is tightly controlled. The acceptable range for absorption is narrow—usually within 80% to 125% of the original. But even within that range, small differences in inactive ingredients can cause unexpected side effects. That’s why drug interactions, unintended reactions between medications or between a drug and something you eat, drink, or take sometimes show up differently with generics. It’s not the active ingredient—it’s the fillers, dyes, or coatings. A pill splitter might save money, but if the coating changes how the drug dissolves, it could throw off your blood levels.
Another big concern is trust. Patients often feel safer with brand names—even when science says otherwise. That’s partly because of confusing batch variability. One batch of a generic might work perfectly, but if the next batch has slightly different manufacturing conditions, it can feel like the drug stopped working. That’s not always the case, but it’s enough to make people anxious. This is where medication safety, the practice of ensuring drugs are used correctly and without harm becomes a team effort. Pharmacists checking for interactions, doctors explaining why a generic is safe, and patients reading the Medication Guide—all of it adds up. The FDA’s black box warnings, reversal agents for overdoses, and even how Europe’s tendering systems enforce quality aren’t just policies—they’re layers of protection built into the system.
You’ll find posts here that cut through the noise. Learn how multiple generic makers don’t always mean lower prices. See why some people react to inactive ingredients in generics but not the brand. Understand how team-based care helps doctors and pharmacists catch risks before they happen. And get real facts on whether biosimilars—often confused with generics—are truly as safe as the originals. This isn’t about marketing. It’s about knowing what’s in your pill, how it behaves, and who’s responsible for making sure it doesn’t hurt you.
Cardiovascular generics save billions, but safety data shows mixed outcomes. Learn what studies really say about effectiveness, risks, and when to stick with brand names.
The FDA requires generic drugs to meet the same safety, strength, and effectiveness standards as brand-name drugs through strict bioequivalence testing and manufacturing oversight. Learn how generics are approved and why they're reliable.
Generic drugs save money but aren't tested like brand names before approval. Learn how the FDA tracks hidden safety issues after they hit the market-and what you can do to stay safe.

This article breaks down nine alternatives to Dexamethasone, focusing on their anti-inflammatory properties and potential benefits. It discusses options like Butyric Acid, which supports gut health while offering natural relief, and touches on both the pros and cons. Comprehensive insights are provided for those interested in alternative therapies.
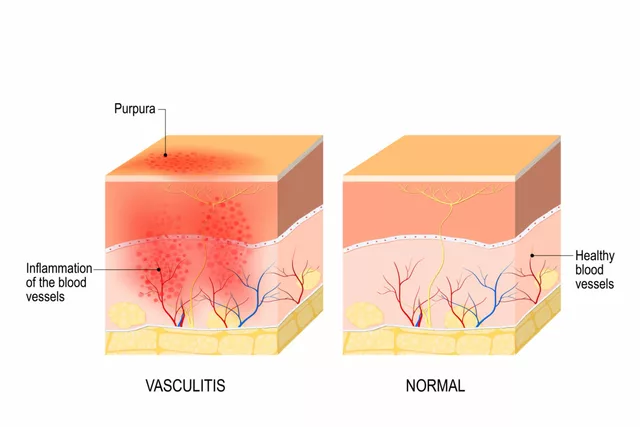
As a blogger, I've recently delved into the topic of Atorvastatin's role in treating vasculitis. Research suggests that this cholesterol-lowering drug may help reduce inflammation in the blood vessels, thus improving the symptoms of this autoimmune disease. Studies have shown promising results, but more research is needed to confirm its effectiveness. Personally, I'm intrigued by the potential of Atorvastatin in treating vasculitis and will be keeping an eye out for further developments. In the meantime, I encourage everyone to discuss treatment options with their healthcare professionals.
Discover the science behind Primidone's anticonvulsant action, its metabolism to phenobarbital, key pharmacokinetic traits, and how it fits into epilepsy therapy.
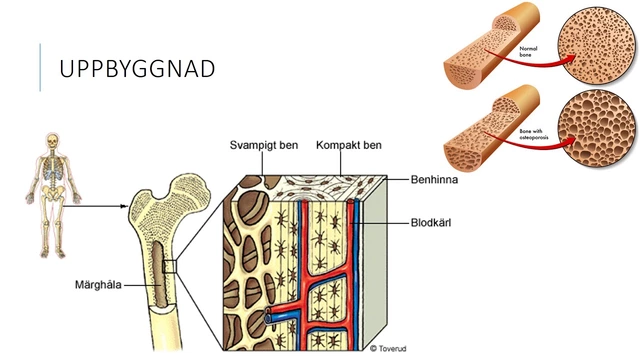
In my recent research on osteoporosis, I discovered that Strontium could be a secret weapon against this debilitating condition. Strontium is a naturally occurring mineral that has been shown to promote bone growth and density. It works by mimicking calcium in our bones, therefore increasing bone strength and reducing the risk of fractures. I was surprised to learn that many people are unaware of this mineral's potential benefits for their bone health. Incorporating Strontium into our daily diet or as a supplement could make a significant difference in our battle against osteoporosis.
A side‑by‑side look at Geriforte and its main competitors, highlighting how they work, costs, effectiveness and who should choose each option.
