More than 9 out of 10 prescriptions filled in the U.S. are for generic drugs. Yet, many patients still hesitate to take them. Why? Because they don’t understand what generics really are. They see a different color, a different shape, a different name - and assume something’s wrong. That’s where infographics about generics come in. These visual tools turn confusing science into clear pictures, helping patients trust their medications - and their care.
What Makes a Generic Drug the Same?
Generic drugs aren’t knockoffs. They’re exact copies in active ingredients, strength, dosage form, and how they work in the body. But that’s not obvious to someone holding a pill that looks nothing like the brand-name version they remember.
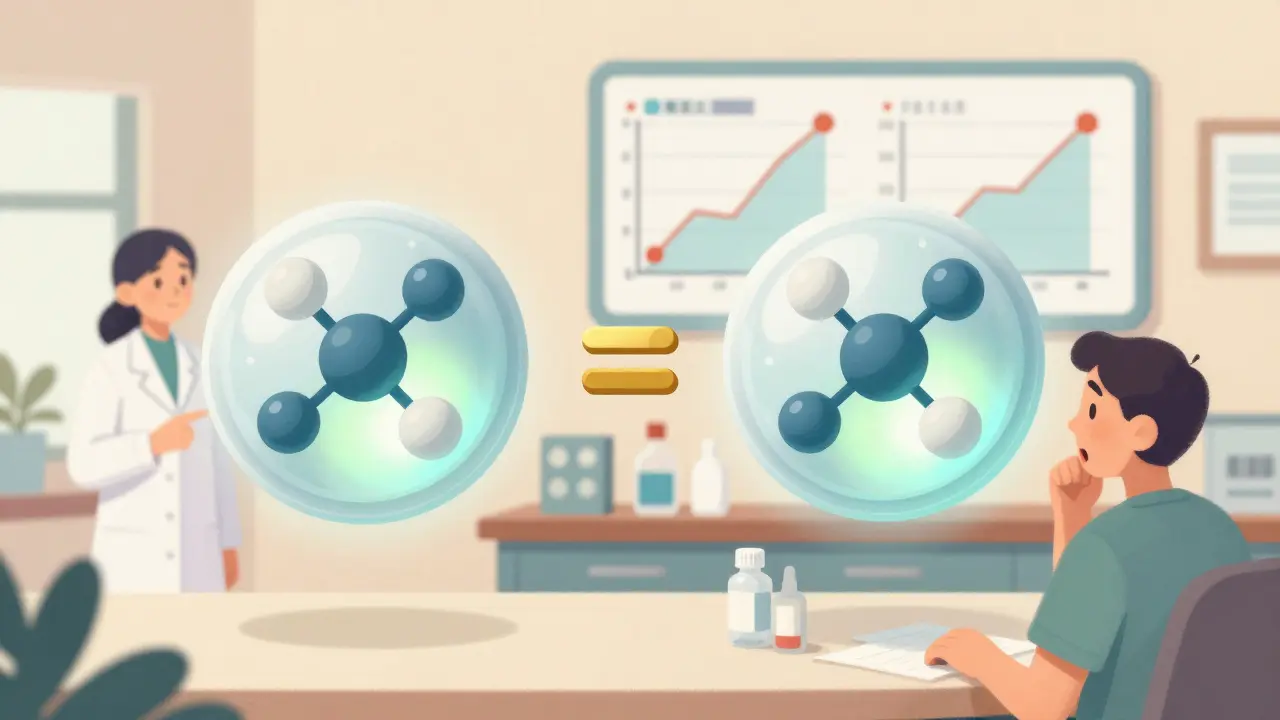
The FDA’s infographic “What Makes a Generic the Same as a Brand-Name Drug?” uses simple visuals to show this. One panel compares the molecular structure of a brand drug and its generic side by side - identical. Another shows dissolution curves: how fast the drug dissolves in the body. The curves overlap perfectly. That’s not luck. It’s required by law.
Patients don’t need to know the word “bioequivalence.” They just need to see that the numbers match. In FDA testing, 89% of people who saw this infographic correctly understood that generics work the same way. That’s a big jump from the 43% of patients who doubted generics in 2021.
How Are Generics Approved?
Some patients think generics are cheaper because they’re lower quality. That’s a myth. The FDA holds generics to the same strict standards as brand-name drugs. The same factories, sometimes the same equipment, often the same teams.
An infographic from the FDA walks through the approval process step by step: ingredient sourcing, lab testing, manufacturing checks, and post-market surveillance. Each step is shown with icons - a beaker, a checklist, a microscope - not paragraphs of text. The message is clear: no shortcuts.
One common question: “If the generic is made in India or China, is it safe?” The infographic doesn’t dodge this. It shows that FDA inspectors visit every facility - domestic or foreign - the same way. And they do it unannounced. No country gets special treatment.
Why Do Generics Cost Less?
Generics don’t cost less because they’re cheaper to make. They cost less because they don’t need to pay for advertising, celebrity endorsements, or 10 years of R&D. That’s the real story.
A visual timeline in the FDA’s “Exclusivity and Generic Drugs: What Does It Mean?” infographic shows how a brand drug gets 12 years of market protection. After that, other companies can apply to make the generic. No more patents. No more monopoly. Prices drop fast.
The numbers speak for themselves. From 2010 to 2019, generics saved the U.S. healthcare system $1.68 trillion. That’s more than the GDP of most countries. Patients don’t need to understand economics. They just need to see that saving money doesn’t mean sacrificing safety.
Who Uses These Infographics?
Pharmacists are the front line. At Kaiser Permanente, 78% of pharmacists use FDA infographics during consultations. One pharmacist said, “I print this and keep it behind the counter. It cuts counseling time in half.”
Doctors use them too. The American Medical Association rated the FDA’s generic drug infographics 4.7 out of 5 for helping explain substitutions to patients. Nurses keep them in waiting rooms. Community health centers hand them out with prescriptions.
But it’s not just clinics. Patients download them directly. In 2022, the FDA distributed 1.7 million copies. About 37% came from consumers searching online. That’s people asking for help on their own - a sign these tools are working.
What’s Missing?
Not all infographics are perfect. Some patients still find the language too technical. Dissolution curves, while accurate, can look like squiggly lines to someone without a science background.
There’s also a gap in equity. African American and Hispanic patients are more likely to worry about generics. But only one FDA infographic - the “Generic Drugs and Health Equity Handout” - directly addresses this. It shows how generics reduce cost barriers for low-income families, rural communities, and uninsured patients.
Another blind spot: drugs with narrow therapeutic indexes - like warfarin or levothyroxine. A small change in how the drug is absorbed can matter. Current infographics don’t clearly warn patients when switching brands might need extra monitoring. The Institute for Safe Medication Practices says this needs a visual flag - maybe a yellow triangle or a pharmacist icon.

How to Use These Tools
You don’t need a degree to use these infographics. Here’s how real people use them:
- Ask your pharmacist for a printed copy when you get a generic prescription.
- Save the PDF on your phone. Open it when you’re confused about why your pill looks different.
- Share it with family members who are also on generics.
- Use it as a conversation starter with your doctor: “I saw this. Can we talk about my meds?”
Hospitals and clinics that use the full FDA Toolkit - including social media posts and info cards - saw a 22% increase in patients accepting generics within six months. That’s not magic. That’s clarity.
What’s Next?
These tools are evolving. In 2023, the FDA released Version 2.0 of its main infographic with updated savings numbers: $313 billion saved annually. That’s $1,000 per person in the U.S. every year.
Interactive versions are coming. The GTMRx Institute launched digital infographics where you can type in your medications and get a personalized breakdown of which ones have generics, which don’t, and how much you could save.
By 2024, the FDA plans to test augmented reality. Point your phone at a pill bottle, and a 3D model of the drug’s molecule appears - brand and generic side by side. No more guessing.
These aren’t just pictures. They’re tools to rebuild trust. And trust saves lives.
Are generic drugs really as safe as brand-name drugs?
Yes. Generic drugs must meet the same strict standards as brand-name drugs set by the FDA. They contain the same active ingredients, work the same way in the body, and are made in the same type of facilities. The FDA inspects both brand and generic drug factories the same way - and they do unannounced checks. There’s no difference in safety.
Why do generic pills look different from brand-name pills?
The appearance - color, shape, size, or markings - doesn’t affect how the drug works. Federal law says generic manufacturers can’t copy the exact look of brand-name pills to avoid trademark issues. But the active ingredient, dosage, and effectiveness are identical. Infographics show this clearly with side-by-side comparisons so you know the difference is only cosmetic.
Can I trust generics made outside the U.S.?
Yes. The FDA inspects all drug manufacturing facilities - whether they’re in the U.S., India, China, or elsewhere - using the same rules. No country gets special treatment. In fact, more than half of all generic drugs sold in the U.S. are made overseas, and they’re held to the same quality standards as those made domestically.
Do generic drugs work slower or weaker than brand-name drugs?
No. Generics must prove they release the active ingredient into the body at the same rate and to the same extent as the brand-name drug. This is called bioequivalence. FDA testing uses precise measurements - like dissolution curves - to confirm this. If the generic doesn’t match within strict limits, it’s rejected. Studies show 89% of patients who see this explained visually understand it correctly.
What should I do if I think my generic isn’t working?
Don’t assume the generic is the problem. Talk to your pharmacist or doctor. Some people notice small differences in side effects because of inactive ingredients (like dyes or fillers), not the active drug. Others may have changed their diet, sleep, or other medications. The FDA’s infographics explain inactive ingredients clearly. If you’re still unsure, ask for a copy of the patient information sheet - it lists every ingredient. Never stop taking your medicine without talking to your provider.
Are there any drugs where generics aren’t recommended?
For most drugs, generics are perfectly safe. But for a small number - like warfarin, levothyroxine, or some seizure medications - even tiny changes in how the drug is absorbed can matter. These are called narrow therapeutic index drugs. While the FDA still approves generics for these, some doctors prefer to stick with one brand. Always ask your provider if your specific medication falls into this category. Newer infographics are starting to include warnings for these cases.
Where can I find these infographics?
The FDA offers all its generic drug infographics for free on its website. Search for “FDA Generic Drugs Infographics.” You can download them as PDFs, print them, or share them digitally. Many pharmacies, clinics, and hospitals also have printed copies available. Some health systems, like Kaiser Permanente and Epic EHR, now include them directly in patient portals.












9 Comments
So let me get this straight: we’re spending billions on marketing pills that look different, but the actual medicine is identical? And we call this capitalism? I mean, if I bought a Toyota and got a Honda that ran the same, I’d be thrilled - but apparently, pills are different. Weird.
I've been a pharmacist for 22 years, and I can tell you - this is the most important tool we've had in decades!!! People don't understand that the color change doesn't mean the drug is weaker - it means the manufacturer didn't pay for the trademarked design!! And yes, the FDA inspects factories in India just as hard as they do in New Jersey - and they show up at 3 a.m. with no warning!!! You think they're cutting corners? They're not. They're just not charging you $12 for a pill that costs $0.30 to make!!!
The notion that bioequivalence is a sufficient proxy for therapeutic equivalence is, frankly, a reductive heuristic that ignores the pharmacokinetic nuances inherent in polymorphic crystalline structures and excipient-mediated absorption dynamics - particularly in narrow-therapeutic-index agents. The FDA’s infographics, while aesthetically pleasing, are dangerously oversimplified.
Let us not forget that trust is not a commodity that can be manufactured through infographics alone - it is the cumulative result of transparency, consistency, and human connection. When a patient holds a pill that looks foreign, they are not merely questioning the chemistry - they are questioning whether the system still cares about them. These visuals are not just educational tools; they are bridges - fragile, vital, and beautifully human - connecting the cold arithmetic of pharmaceutical economics to the warm, trembling hope of someone who just needs to feel safe again.
My grandma used to freak out every time her blood pressure med changed shape. I printed the FDA infographic and taped it to her pillbox. She still asks about it every month. ‘Is this the same one?’ she says. And I say, ‘Yep. Same magic, different wrapper.’ She smiles. That’s all we need sometimes.
I used to be terrified of generics - I thought they were like knockoff sneakers: same shape, but the glue falls out after a week. But then I saw that infographic with the side-by-side dissolution curves - it looked like two identical waves crashing on the same shore. I cried. Not because I was moved by science - but because I realized I’d been scared of a ghost. The pill didn’t change. My fear did. And now? I save $80 a month. I feel like I won the lottery - without even buying a ticket.
Oh, darling, this is just another capitalist illusion wrapped in pastel icons - ‘trust the system!’ they say, while the FDA inspector sips espresso in a five-star hotel after inspecting a factory in Gujarat. And yet - I still take my generics. Because what choice do we have? The system is a theater, and we are all actors in a play where the script was written by shareholders and printed on recycled paper. But oh, how I love the lighting!
My cousin’s kid has epilepsy. They switched generics once - and had a seizure. The doctor said it was coincidence. But I’ve seen it too many times. These infographics? Cute. But they don’t fix the fact that some people are just more sensitive. You can’t infographic your way out of biology.
Okay so like i was reading this and i think its so cool that the FDA does all these checks and stuff and the pills are the same but wait - what about when you switch from one generic to another generic? Like i got my levothyroxine from one company and then the pharmacy switched it and i felt like i was on a rollercoaster for two weeks?? i mean the infographic says it’s the same but like… it felt different?? so maybe the infographic needs a big yellow triangle that says ‘this one might make you feel weird for a bit’?? also i think the FDA should do a meme campaign with a dancing pill??