FDA Generic Drug Quality: What You Need to Know About Safety and Consistency
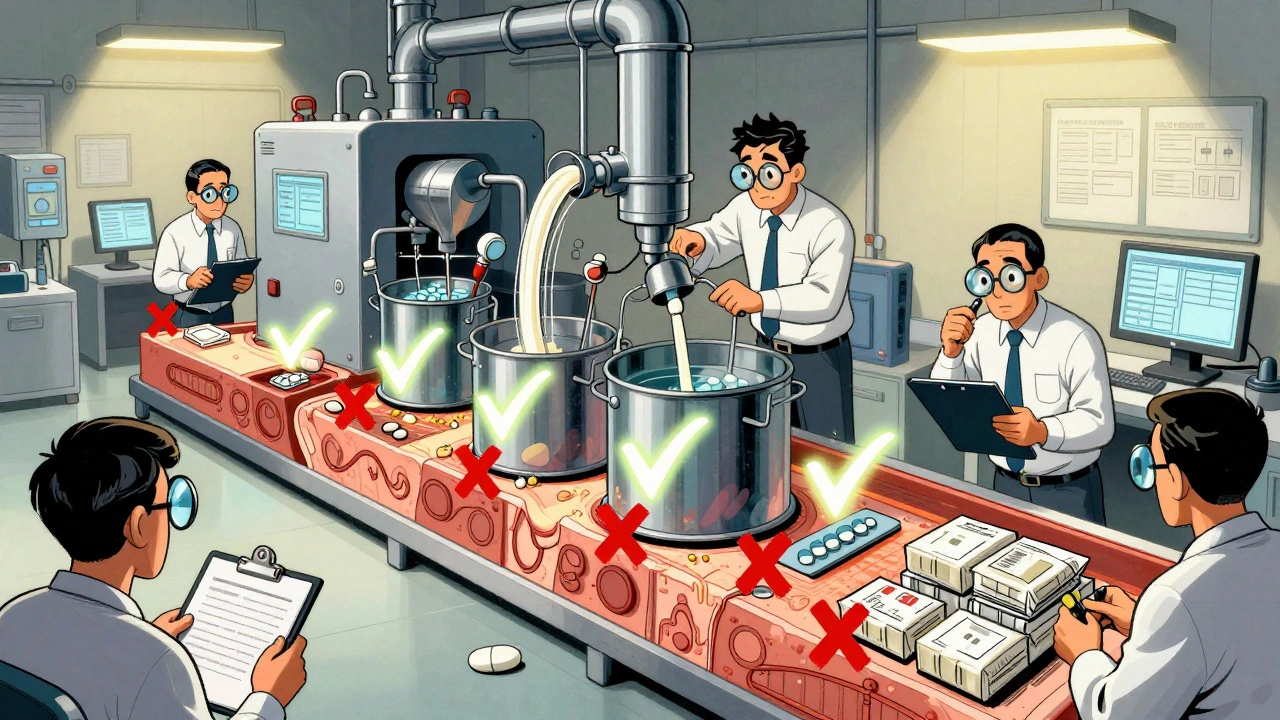
When you pick up a generic pill, you expect it to work just like the brand-name version—and the FDA generic drug quality, the set of standards and monitoring systems the U.S. Food and Drug Administration uses to ensure generic medications are safe, effective, and consistent. Also known as bioequivalence standards, it’s not just about matching the active ingredient. It’s about making sure every batch, from every factory, delivers the same result in your body. The FDA doesn’t require new clinical trials for generics. Instead, they rely on rigorous lab tests to prove the drug breaks down the same way, gets absorbed at the same rate, and reaches the same level in your bloodstream. That’s the core of bioequivalence, the scientific measure that proves a generic drug performs the same as its brand-name counterpart. But what happens after approval? That’s where things get real.
Here’s the catch: a drug can meet bioequivalence standards and still have problems later. batch variability, differences in how a drug performs from one production run to the next. Even tiny changes in filler ingredients, manufacturing conditions, or tablet coating can affect how quickly your body absorbs the medicine. One batch might work perfectly. The next? You might feel dizziness, nausea, or notice your blood pressure isn’t as controlled. That’s not rare—it’s underreported. The FDA tracks these issues through post-market surveillance, the ongoing monitoring of drugs after they’re sold to the public. This system relies on reports from patients, doctors, and pharmacists. If enough people report the same issue, the FDA can demand changes, recall batches, or even pull the drug.
And it’s not just about the active drug. Sometimes, the problem isn’t the medicine itself—it’s the other stuff in the pill. Inactive ingredients like dyes, binders, or preservatives can trigger allergies or interact with other meds. That’s why some people swear their generic version doesn’t work like the brand—even when science says they’re identical. Trust isn’t just built on data. It’s built on experience. That’s why understanding FDA generic drug quality means knowing the system has gaps, and knowing how to spot when something’s off.
What you’ll find below are real stories and hard facts about how generics are made, tested, and monitored. From how one batch can differ from another to how patient reports change drug safety rules, these posts give you the tools to ask better questions, spot warning signs, and take control of your meds—no matter the price tag.