Generic Drug Monitoring: What You Need to Track for Safety and Effectiveness
When you take a generic drug, a chemically identical version of a brand-name medication approved by regulators like the FDA or EMA. Also known as brand-equivalent medication, it’s meant to save you money without sacrificing results. But here’s the catch: getting the same active ingredient doesn’t always mean you’ll get the same experience. That’s where generic drug monitoring, the ongoing process of tracking how a generic medication performs in real-world use comes in. It’s not about trusting labels—it’s about watching for changes in how you feel, how your blood tests respond, or if side effects suddenly appear.
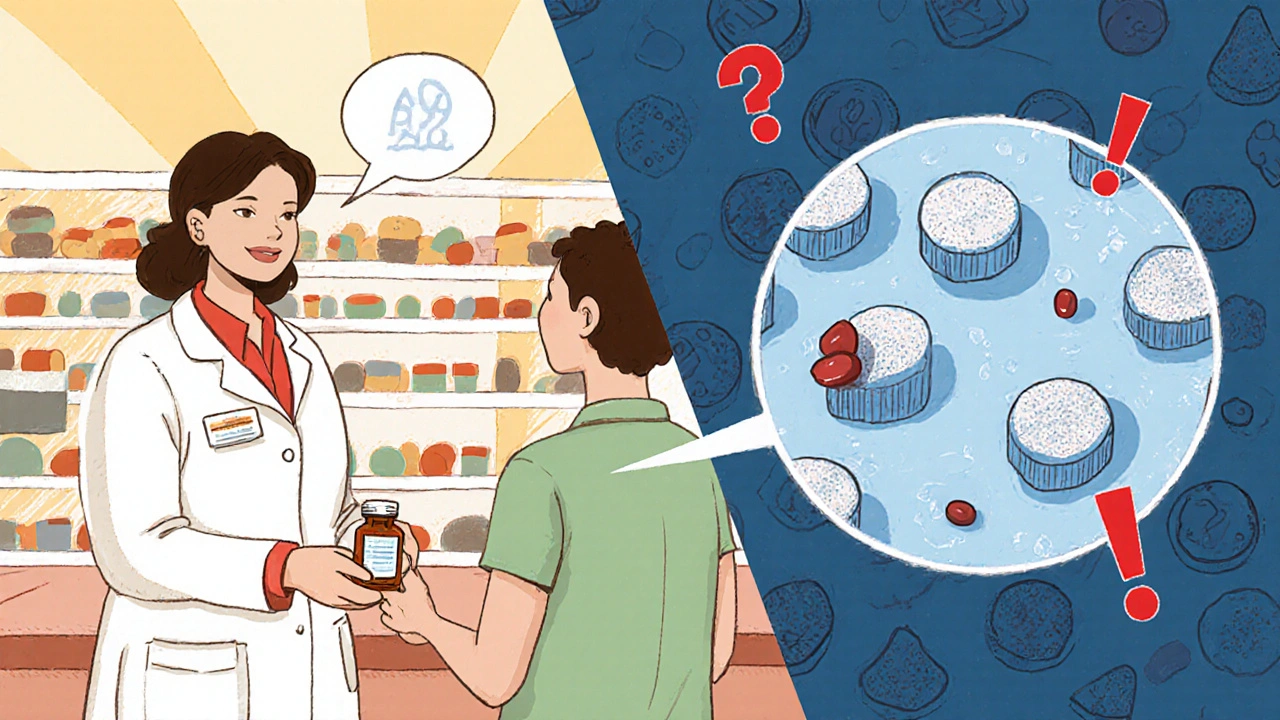
Why does this matter? Because bioequivalence, the standard that proves a generic drug behaves like the original in your body is tested in healthy volunteers under perfect conditions. Real life? You’re taking it with food, other meds, or after a bad night’s sleep. That’s where drug interactions, unexpected reactions between your generic pill and something else you’re taking can sneak in—even if the active ingredient hasn’t changed. A different filler in the pill might slow absorption. A new batch might dissolve slower. These aren’t errors—they’re normal variations under allowed limits. But if you’re on a blood thinner, an antiseizure drug, or a heart medication, even small shifts can be dangerous.
People think generics are all the same. They’re not. One person might switch from brand to generic and feel fine. Another might get dizzy, nauseous, or see their INR spike. That’s why monitoring isn’t optional—it’s personal. Track your symptoms. Keep a log of when you refill. Note any changes in how you feel after a new pharmacy or a new bottle. Talk to your pharmacist when the pill looks different. Ask if the manufacturer changed. These aren’t paranoid questions—they’re smart ones.
And it’s not just about you. medication safety, the system of practices and checks designed to prevent harm from drugs depends on people speaking up. When you notice something off, you help build the data that protects others. The FDA and EMA don’t track every batch. But when enough patients report the same issue—like a generic blood pressure pill causing more dizziness than before—that’s when regulators step in.
You don’t need to be a scientist to monitor your meds. You just need to pay attention. If you’ve ever wondered why your generic thyroid pill suddenly didn’t work as well, or why your cholesterol jumped after switching brands, you’re not alone. The answers are often in the details—timing, diet, batch numbers, and how your body reacts. The posts below show you exactly how to spot red flags, what tests to ask for, how to talk to your doctor without sounding suspicious, and which generics are more likely to cause trouble. No fluff. No jargon. Just what you need to know to stay safe while saving money.