FDA pharmaceutical quality: What It Means and Why It Matters
When you pick up a pill from the pharmacy, whether it’s brand-name or generic, you trust that it will work the same way every time. That trust comes from FDA pharmaceutical quality, the set of rules and inspections the U.S. Food and Drug Administration uses to ensure every drug meets strict safety, strength, and consistency standards. Also known as drug manufacturing compliance, it’s not just paperwork—it’s what stops contaminated, weak, or mismatched medicines from reaching you.
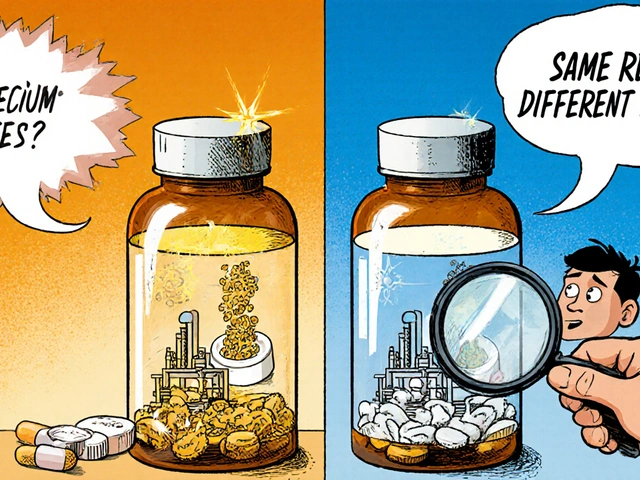
This system doesn’t stop at approval. The FDA keeps watching drugs after they hit the market through post-market surveillance, a continuous monitoring process that tracks real-world side effects, manufacturing issues, and unexpected interactions. This is how the FDA catches problems that clinical trials miss—like a batch of generic blood pressure pills that doesn’t dissolve properly, or a new interaction between a common antibiotic and a heart medication. These aren’t hypothetical risks; they’re real events logged in the FAERS database and investigated by FDA scientists. And when it comes to generics, the biggest concern isn’t whether they work—it’s whether every single batch performs the same. That’s where bioequivalence, the scientific standard that proves a generic drug releases its active ingredient at the same rate and amount as the brand version. Also known as pharmacokinetic equivalence, it’s the backbone of why a $5 generic can replace a $100 brand-name drug without sacrificing safety. But bioequivalence isn’t perfect. Batch variability can still happen, and the FDA’s limits on that variability are constantly being reviewed as new data comes in.
It’s not just about the active ingredient. Inactive ingredients—like fillers, dyes, or coatings—can cause reactions in sensitive people. That’s why the FDA tracks reports of allergic responses and unexpected side effects tied to specific manufacturers. If a generic version of a drug starts causing more rashes or dizziness than the brand, the FDA investigates the formulation. This is the same system that caught unsafe levels of NDMA in some blood pressure meds, leading to massive recalls. You don’t see the inspections, but they’re happening in factories across the U.S. and around the world.
What you’ll find in the posts below is a practical look at how FDA pharmaceutical quality affects real patients. From how generic drug competition can sometimes hurt supply chains, to why batch variability matters more than you think, to how post-market studies reveal hidden risks—every article ties back to one truth: drug safety isn’t a one-time check. It’s a constant, invisible effort that keeps you protected. You won’t find fluff here. Just clear facts on what the FDA does, what it misses, and what you can do to stay safe.