When you’re dealing with stomach ulcers, acid reflux, or irritable bowel issues, doctors often prescribe gastrointestinal combination products-medicines that pack two or more active ingredients into one pill. The idea is simple: fewer pills, better results. But as these drugs age, generics start showing up, and suddenly, your prescription might change-or cost a lot less. Knowing what’s available, what’s not, and what alternatives exist can save you money and keep your treatment on track.
What Are Gastrointestinal Combination Products?
These aren’t just random mixes of pills. They’re carefully designed to work together. For example, a common combo for H. pylori infection includes a proton pump inhibitor (PPI) like omeprazole plus two antibiotics: amoxicillin and clarithromycin. The PPI lowers stomach acid so the antibiotics can kill the bacteria more effectively. Without the acid control, the antibiotics might not work as well. Another example is Duexis, which combines 800 mg of ibuprofen (a painkiller and anti-inflammatory) with 26.6 mg of famotidine (an acid reducer). This combo is approved specifically for people with arthritis who also need protection from stomach ulcers caused by long-term NSAID use. It’s not just convenience-it’s safety. Newer combinations are popping up too. In 2024, the FDA approved vonoprazan (brand name Voquezna), a potassium-competitive acid blocker that works faster and lasts longer than traditional PPIs. It’s used for heartburn from nonerosive GERD. Unlike PPIs, it doesn’t need to be activated by stomach acid, so it starts working almost immediately.Generic Availability: What’s Available and What’s Not
The good news? Many older combination products now have generic versions. The bad news? Not all of them do-and timing matters. For instance, the ibuprofen-famotidine combo (Duexis) got its first generic approval in August 2021. Par Pharmaceutical and Alkem Laboratories Limited both launched generic versions. That means if your doctor prescribes Duexis now, you’ll likely get the generic unless there’s a specific reason not to. The generic works the same way, costs less, and is FDA-approved as therapeutically equivalent. Linzess (linaclotide), used for constipation-predominant IBS, also has a generic version approved in 2021 by Mylan. So if you’re on that, you’re probably already on a generic unless your insurance requires the brand. But here’s where it gets tricky. Some combinations still don’t have generics. Vonoprazan, approved in July 2024, is still under patent protection. That means it’s only available as the brand-name Voquezna-for now. The same goes for newer biologics like risankizumab-rzaa (Pyzchiva), a biosimilar to Stelara used for Crohn’s and ulcerative colitis. Biosimilars are cheaper than original biologics, but they’re not generics. They’re highly similar, but complex to copy. And then there’s Xifaxan (rifaximin), which lost exclusivity in 2024 after 20.7 years on the market. That means generics are coming soon. But even when a generic is approved, it doesn’t mean it’s immediately available at your pharmacy. Manufacturers sometimes delay production. Always check with your pharmacist if your prescription isn’t filling as expected.Why Some Combinations Are Harder to Genericize
Not all pills are created equal. Some combinations have complex formulations that make generic versions harder to produce. Take H. pylori triple therapy: even though omeprazole, amoxicillin, and clarithromycin are all available as generics, the exact dosage ratios and timing matter. If you take them separately, you have to remember three pills at different times. The combo pill ensures you get the right dose at the right time. Also, some drugs have patent extensions or legal battles that delay generics. For example, Janumet (sitagliptin + metformin) is expected to face generic competition in 2026. But because it’s a diabetes drug used for GI-related issues like gastroparesis, it’s still relevant here. The FDA treats combination drugs as separate entities from their single-ingredient versions. So even if metformin has been generic for years, Janumet was protected as its own drug. This matters for insurance too. Medicare and Medicaid often negotiate prices separately for each combination. That means a generic version of Janumet won’t automatically bring down the price of Januvia (sitagliptin alone). They’re treated like different drugs, even if they share an ingredient.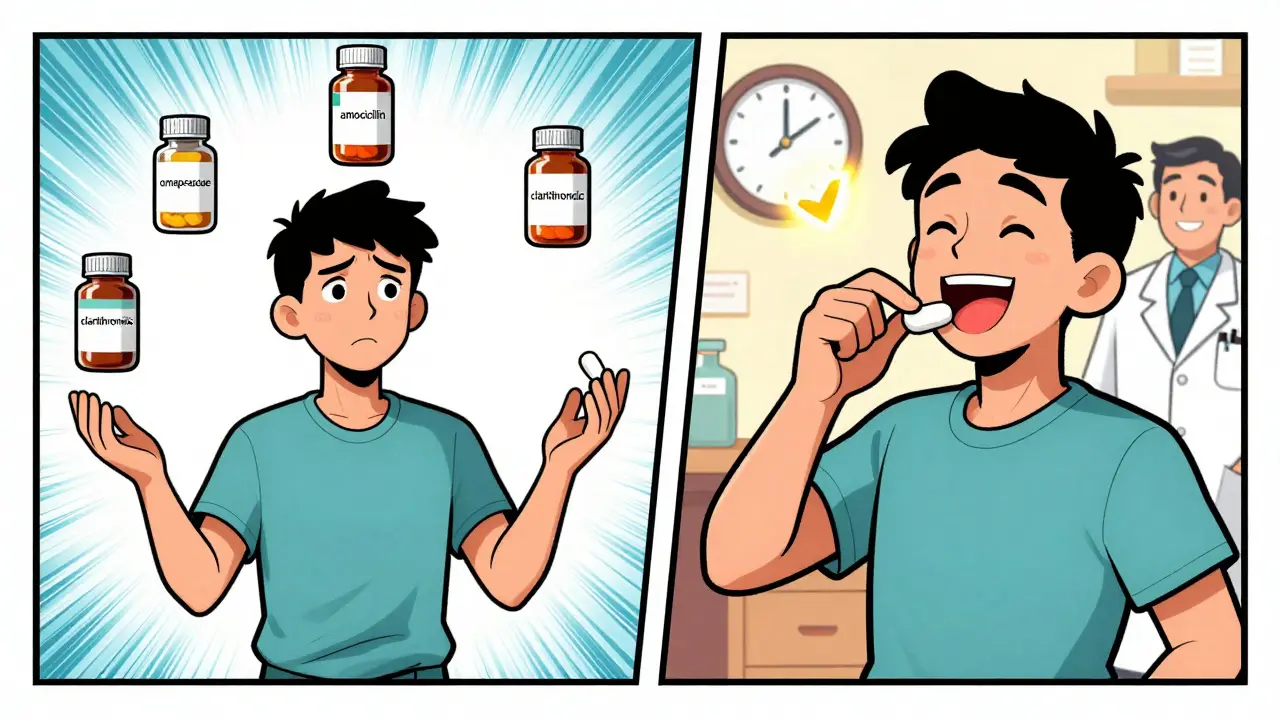
Alternatives to Combination Products
If your combination product doesn’t have a generic-or if you can’t get it covered-there are alternatives. One common approach is to take the individual ingredients separately. For example, instead of Duexis, you could take a generic ibuprofen tablet and a generic famotidine pill. This gives you the same active ingredients, but you’ll have to manage two pills. Some people prefer this because they can adjust doses independently. But if you forget one, you lose the protection. For acid reflux, if vonoprazan is too expensive, you can still use generic omeprazole, esomeprazole, or pantoprazole. These are all PPIs and work well for most people. The difference? Vonoprazan works faster and may be better for people who don’t respond to PPIs. But for mild GERD, the older generics are still first-line. For H. pylori, if you can’t get the triple combo pill, your doctor can prescribe the three components separately. The key is making sure you take them correctly: usually twice a day for 10-14 days. Missing doses lowers your chance of curing the infection. There’s also over-the-counter options. Loperamide (Imodium) is available OTC for diarrhea. It’s not a combination product, but it’s often used alongside other GI meds. For occasional heartburn, antacids like Tums or Pepcid AC can help-but they’re not for long-term use.Insurance, Prior Authorization, and Access Hurdles
Even when generics exist, insurance can make things complicated. Many plans require prior authorization (PA) for brand-name combination drugs. For example, if you’re on a high-dose PPI like omeprazole 40 mg twice daily, your insurer might ask for proof that you tried generics first or that you have a condition like Zollinger-Ellison syndrome. MassHealth and other Medicaid programs have strict rules. If you want the brand-name version of a drug that has a generic equivalent, you need documentation showing you had an adverse reaction or that the generic didn’t work. That means your doctor has to write a letter explaining why the brand is necessary. The flip side? Generic versions often don’t need PA. That’s why many patients switch to generics when they can. But if you’ve been on a brand for years and your doctor says you respond better to it, you can appeal. Many patients do-especially if they’ve had side effects with the generic.
What to Do If Your Medication Changes
If your pharmacy switches your prescription from a brand to a generic without telling you, check the label. The active ingredients should match exactly. If you notice new side effects-like more stomach upset, dizziness, or diarrhea-talk to your doctor. Not everyone reacts the same to generics, even if they’re technically equivalent. Also, ask your pharmacist if the generic you’re getting is from a reputable manufacturer. Some generic versions are made overseas and may have inconsistent quality. The FDA approves them, but real-world experience varies. If you’re unsure whether a generic is right for you, ask for a 30-day trial. Many doctors will let you test the generic before switching permanently.What’s Coming Next?
The GI drug market is growing fast-projected to hit $96 billion by 2035. More biologics and novel combos are in the pipeline. Drugs like Livmarli (maralixibat), approved in 2024 for rare liver conditions causing severe itching, show how targeted these treatments are becoming. Expect more generics to hit the market in 2025-2026. Janumet, Pomalyst, and Xifaxan are all due for generic competition. That could bring down prices significantly. At the same time, newer drugs like vonoprazan and biosimilars like Pyzchiva will stay expensive for now. But as more patients need long-term GI care, pressure will grow to make these drugs affordable.Bottom Line: Know Your Options
Gastrointestinal combination products are powerful tools-but they’re not the only option. Generics are available for many of the older combos, and taking individual ingredients separately can work just as well if you’re careful. If your insurance denies coverage, ask for a prior authorization form. If you’re paying out of pocket, compare prices at different pharmacies. Some online pharmacies or discount programs like GoodRx can cut your bill in half. And if you’re unsure whether you need a combo product at all, talk to your doctor. Sometimes, simpler treatments-like diet changes, stress management, or single-agent meds-are enough. You don’t always need a two-in-one pill to feel better.Are there generic versions of Duexis (ibuprofen and famotidine)?
Yes. Generic versions of Duexis, containing 800 mg ibuprofen and 26.6 mg famotidine, were approved by the FDA in August 2021. Manufacturers like Par Pharmaceutical and Alkem Laboratories Limited now produce these generics. They are FDA-rated as therapeutically equivalent (A-rated), meaning they work the same as the brand name. Most insurance plans cover the generic at a lower cost.
Can I take ibuprofen and famotidine separately instead of using a combo pill?
Yes, you can. Many people take generic ibuprofen and generic famotidine as separate pills. This gives you more control over dosing-for example, you can skip the famotidine on days you don’t take ibuprofen. The downside is remembering to take two pills instead of one. For people with arthritis who need daily pain relief and stomach protection, the combo pill improves adherence.
Why is vonoprazan (Voquezna) so expensive and not available as a generic?
Vonoprazan was approved by the FDA in July 2024 and is still under patent protection. It belongs to a new class of drugs called potassium-competitive acid blockers (P-CABs), which work differently than older proton pump inhibitors. Because it’s a newer drug with unique chemistry, it hasn’t yet faced generic competition. Generic versions won’t be available until the patents expire, likely in the late 2020s.
Does Medicare cover combination GI drugs differently than single-ingredient drugs?
Yes. Medicare treats combination drugs as distinct entities. For example, Janumet (sitagliptin + metformin) is negotiated separately from Januvia (sitagliptin alone) or metformin. This means even if metformin is cheap and generic, Janumet may still be expensive. The same applies to H. pylori combos-each unique blend is evaluated separately for pricing and coverage under Medicare Part D.
What should I do if my insurance won’t cover my GI combination drug?
First, ask your doctor to submit a prior authorization request. They’ll need to explain why the combination is medically necessary-especially if a generic is available. If that fails, ask about patient assistance programs from the manufacturer. You can also check GoodRx or RxSaver for cash prices, which are sometimes lower than your insurance copay. For older combos like ibuprofen-famotidine, switching to the generic or taking the ingredients separately often resolves coverage issues.









13 Comments
Really appreciate this breakdown. I’ve been on Duexis for years due to arthritis and acid issues, and switching to the generic last year saved me over $200/month. No difference in how I feel-just cheaper. Pharmacies sometimes try to push the brand unless you ask for the generic outright.
Same here. My doc just switched me to generic ibuprofen + famotidine and I didn’t even notice. The combo pill was just a marketing gimmick honestly. Why pay more when the ingredients are the same? People overthink this stuff.
Generic or brand, it’s all about what works for your body. I tried the generic version of vonoprazan when it first came out and got terrible headaches. Went back to Voquezna and boom, no more issues. Don’t assume generics are always better-your system might react differently
Why do Americans pay so much for pills? In India, we get triple therapy for H. pylori for under $5. Everything here is overpriced because of greedy pharma. Your system is broken.
It's important to recognize that therapeutic equivalence does not always translate to clinical equivalence. While generics are bioequivalent per FDA standards, individual patient variability-including absorption kinetics and excipient sensitivity-can lead to differential outcomes. Always monitor for subtle changes in symptom control post-switch.
Don’t let the FDA fool you. Just because it’s ‘A-rated’ doesn’t mean it’s the same. I’ve seen generics with weird fillers that give me bloating. And don’t even get me started on the Chinese-made ones-no wonder people get sick. Stick with the brand if you can afford it. Your gut isn’t a lab rat.
Thank you for this!! 🙌 I was terrified to switch from Duexis but now I’m saving $150/month and my stomach’s fine. Pro tip: Use GoodRx-sometimes the cash price is lower than your copay even with insurance!
Most of you are missing the point. The real issue isn’t generics-it’s that the FDA approves these combos as if they’re novel drugs. It’s regulatory capture. A simple mix of two generics shouldn’t be treated as a patentable entity. This isn’t medicine-it’s corporate arithmetic.
bro in india we just take omeprazole + amoxicillin + metronidazole all separate and its like 200 rupees total. no one cares about combo pills here. why do u need 1 pill when u can take 3? also vonoprazan is just fancy omeprazole with a new name lol
It’s funny how we treat medicine like it’s a one-size-fits-all solution. But our guts are as unique as our fingerprints. What works for one person’s H. pylori might be useless for another’s reflux. Maybe the real innovation isn’t the pill-it’s listening to what your body tells you after the switch.
you guys are so naive. the generic is always worse. i used to take the brand duexis and felt fine. switched to generic and got diarrhea for two weeks. the fillers are toxic. also vonoprazan is the future and youll all be begging for it in 5 years when your ppi stopped working
Just got my Rx switched to generic vonoprazan (yes, it’s still brand) and my insurance denied it-said I need to try PPIs first. So now I’m on pantoprazole + sucralfate. Not ideal, but I’ll take it. Anyone else dealing with this PA nightmare?
Been on the generic Duexis for 8 months. No issues. My pharmacist said the maker is based in Ohio. I trust that more than some overseas plant. Just check the label. If it works, don’t fix it.